
Starting next week, Calyx Global will be assessing carbon credits for nitrous oxide (N2O) abatement in the production of nitric acid and caprolactam. Nitric acid and caprolactam are chemical compounds used in manufacturing industries that produce N2O as a byproduct. This blog series dives into this project type, focusing on nitric acid production as it is more common.
The largest source of N2O emissions in the chemical process industries is the production of nitric acid, accounting for approximately 50% of the total greenhouse gas emissions from this sector. It is estimated that there are between 400 and 600 nitric acid production plants worldwide[1]. N2O abatement is only adopted in less than 50% of plants in developing countries, excluding China.
Nitric acid production is still one of the largest anthropogenic sources of N2O emissions[2], despite the existence of abatement technologies. Some parts of the world have widely implemented N2O abatement in their nitric acid plants, such as countries in the European Union (EU), while significant emitters such as the United States and Russia have not fully embraced the technology. It is believed that the widespread adoption of abatement technologies may significantly reduce global N2O emissions. Carbon revenue has been pursued as a financing mechanism to facilitate the implementation of the technology and has produced over 20 million carbon credits to date through various standards[3].

The image above [4] features a close-up of a nitric acid plant in South Africa
What is nitric acid?
Nitric acid is used to produce explosives, plastic, liquid-fueled rockets and dyes. However, it is primarily used to make synthetic nitrogen-based fertilizers[5]. Such fertilizers have been essential in improving agricultural efficiency to meet the demands of feeding a growing population of over eight billion people[6]. These fertilizers are estimated to support feeding 48% of the world's population, which illustrates the global importance of nitric acid[6]. Nitric acid production for synthetic nitrogen-based fertilizers will likely increase as the global population grows. As nitric acid production expands, corresponding N2O emissions will increase.
Rising global levels of N2O
Prior to the Industrial Revolution, the atmospheric concentration of N2O was 270 parts per billion (ppb)[7]. As of 2023, the global atmospheric concentration has risen to 337 ppb and has risen at a mean growth rate of ~1.0 ppb per year over the past 10 years[8]. The increasing concentration in the air, coupled with its potency, makes nitrous oxide an important target for strategies to abate greenhouse gas emissions.
Global atmospheric N2O levels
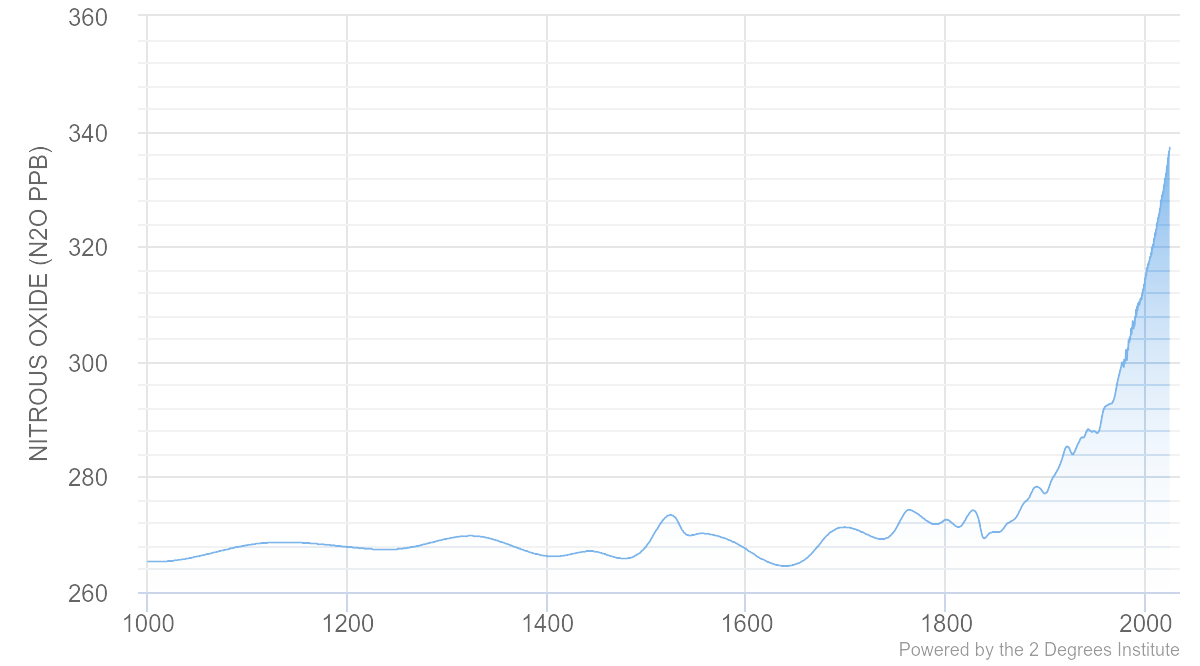
The figure above illustrates how N2O levels have fluctuated in the last 1000 years. Since the Industrial Revolution there has been a dramatic increase in the global atmospheric levels of N2O.
More than CO2: What is GWP?
A chemical’s Global Warming Potential (GWP) represents its warming effect on the atmosphere in units of carbon dioxide equivalence (CO2e). In general, methodologies in the carbon market focus on the GWP over 100 years, partly because the next 100 years will be crucial to our climate future.
According to the IPCC's sixth assessment report, nitrous oxide is a potent greenhouse gas with a GWP estimated to be 273 times greater than CO2 on a 100-year timescale. This means it is 273 times more potent than CO2 at trapping heat in the atmosphere[9] over 100 years*. 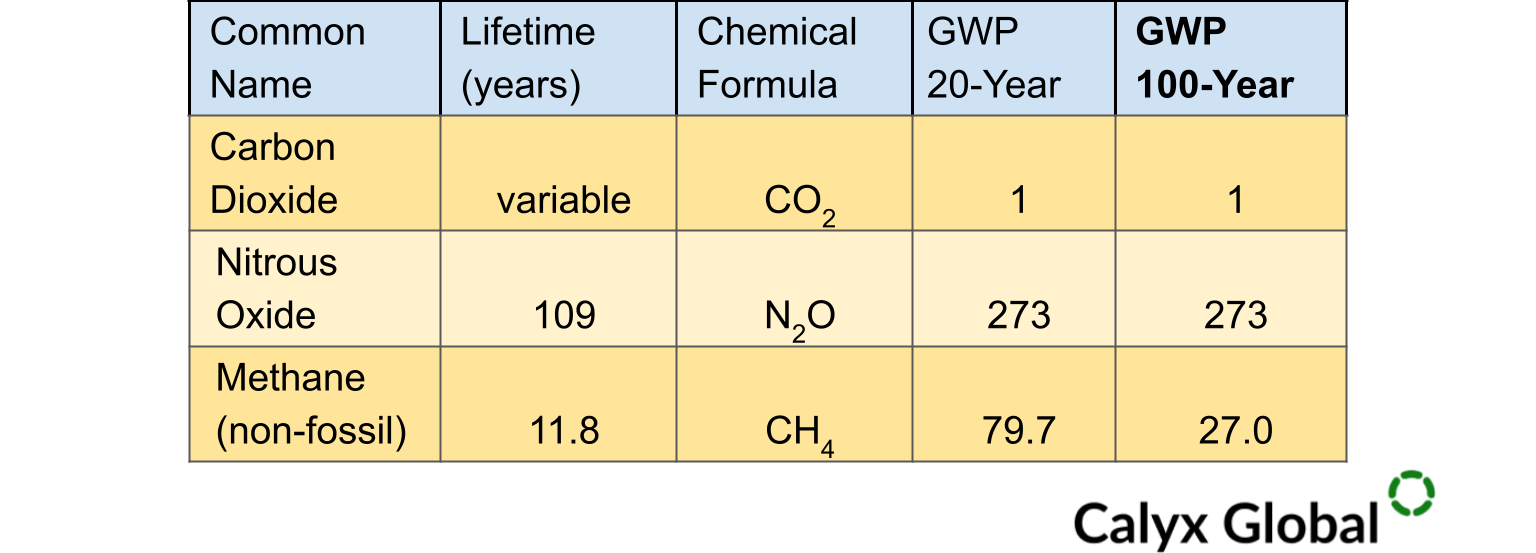
The table above[10] summarizes GWP for carbon dioxide vs. other key non-CO2 GHG. After 100 years, N2O holds 273 times as much heat as CO2 in the atmosphere.
In the carbon market today, the warming impacts of non-CO2 greenhouse gas emissions, such as N2O, are equated to the tonnes of CO2 (tCO2e) that would produce a similar warming impact over 100 years. Carbon credits use this equivalence because each credit represents one tonne of CO2 abatement. One tonne of abated nitric acid results in 273 tCO2e, or 273 carbon credits.
What is N2O abatement in nitric acid plants?
The process of manufacturing nitric acid starts with transforming ammonia (NH3) into nitric oxide (NO), then nitrogen dioxide (NO2), and finally into nitric acid (HNO3). During this process, nitrous oxide (N2O) is formed as an unintentional by-product in the ammonia burner. Apart from some possible losses due to high-temperature decomposition, the nitrous oxide leaving the ammonia burner takes no further part in the chemistry of the nitric acid process and is freely emitted into the atmosphere.
As nitric acid plants are point sources for N2O emissions they make good candidates for the implementation of cost-effective greenhouse gas emission reduction technologies. N2O abatement in nitric acid production is performed through catalytic abatement which essentially increases the efficiency of the nitric acid facility. This process is very similar to what catalytic converters do for our cars. Catalytic converters in our vehicles convert toxic gases into less toxic pollutants in our vehicle’s exhaust pipes.
Catalytic abatement of N2O in nitric acid plants commonly occurs in two ways:
- Secondary Abatement - Nitrous oxide, once formed, is removed anywhere between the outlet of the ammonia oxidation and the inlet of the absorption tower with the implementation of a catalyst.
- Tertiary Abatement - N2O is removed from the tail gas downstream of the absorption tower.
Abatement technologies do not completely eliminate N2O emissions from the source but can achieve emission removal efficiencies of 80% to 98%[12]. Neither abatement type impacts the quantity and quality of the nitric acid production or forms other environmentally undesirable by-products.
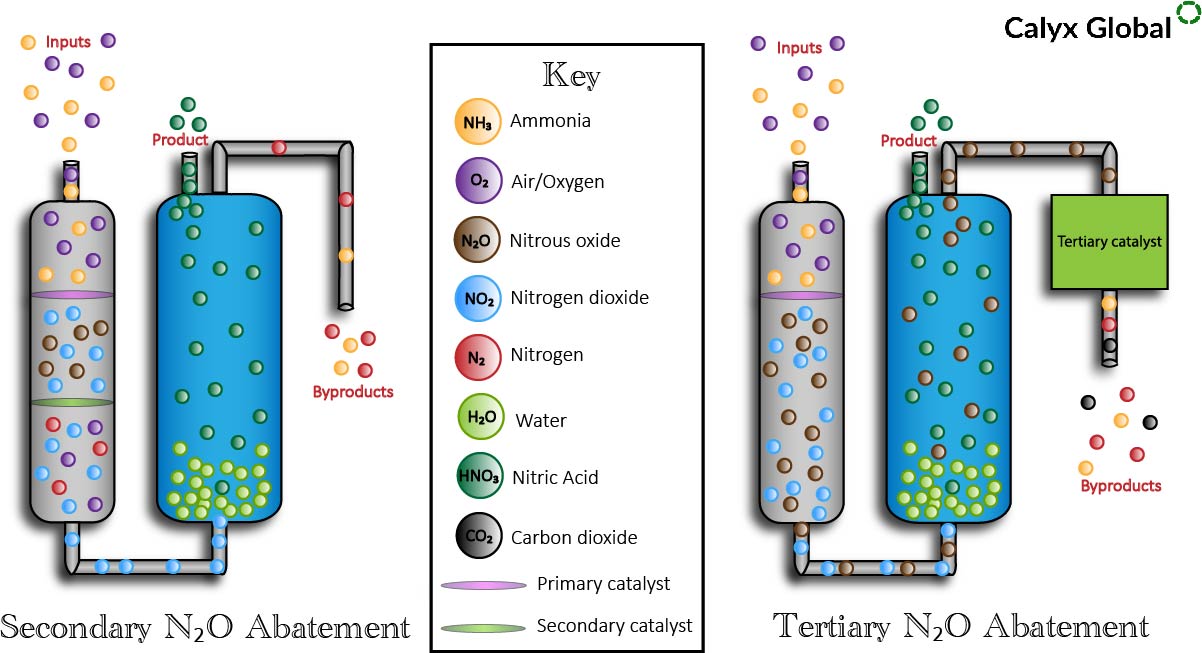
Primary N2O abatement occurs in all nitric acid plants; however, a secondary or tertiary catalyst can be added to the nitric acid production process to further abate N2O emissions. The secondary and tertiary catalysts are placed in different parts of the production system and the technologies work differently, but achieve a similar end result. An additional result of tertiary abatement, which does not occur in secondary abatement processes, is the emission of carbon dioxide.
Stay tuned
The next two blogs in this series will dive into the greenhouse gas (GHG) integrity and potential environmental and social risks of nitric acid projects. We will also highlight what Sustainable Development Goals (SDGs) contributions are possible with this project type.
Calyx Global ratings of N2O abatement projects
Calyx Global has developed a rating framework to assess the GHG integrity of N2O abatement from nitric acid production projects and will be expanding our assessments of this project type. If you’re interested in viewing our ratings of N2O abatement projects, contact us about a subscription to the Calyx Global Platform.
Follow us on LinkedIn, or sign up for our Newsletter below to ensure you do not miss additional content on nitric acid carbon projects.* Note that GWP values change over time due to changes in the atmosphere. The most recent GWP value for N2O is 273, but previous time ranges had different GWPs, as determined in past IPCC reports.
Citations
[1] Kollmuss, A. and Lazurus, M. (2010). Industrial N2O projects under the CDM: The case for nitric acid production. Stockholm Environmental Institute.
[2] Davidson, E. A., & Kanter, D. (2014). Inventories and scenarios of nitrous oxide emissions. Environmental Research Letters, 9(10), 105012. https://doi.org/10.1088/1748-9326/9/10/105012
[3] Ivy S. So, Barbara K. Haya, Micah Elias. (2023, December). Voluntary Registry Offsets Database, Berkeley Carbon Trading Project, University of California, Berkeley.
[4] United Nations online platform for voluntary cancellation of certified emission reductions (CERs). Picture of N2O abatement project at nitric acid plant No. 11 at African Explosives Ltd. (AEL), South Africa, N2O abatement project at nitric acid plant No. 11 at African Explosives Ltd. (AEL), South Africa. 2024 United Nations Framework Convention on Climate Change. https://offset.climateneutralnow.org/n2o-abatement-project-at-nitric-acid-plant-no-11-at-african-explosives-ltd-ael-south-africa-1364-
[5] Nascimento, G.R.O. and Dangelo, J.V.H. (2024). Operating parameters analysis of a nitric acid plant to increase production and reduce NOx gases emission. Chemical Engineering and Processing - Process Intensification. 196.
[6] Erisman, J., Sutton, M., Galloway, J. (2008). How a century of ammonia synthesis changed the world. Nature Geosci 1, 636–639.
[7] Liang, M., Zhou, Z., Ren, P., Xiao, H., Xu-Ri, Hu, Z., Piao, S., Tian, H., Tong, Q., Zhou, F., Wei, J., & Yuan, W. (2023). Four decades of full-scale nitrous oxide emission inventory in China. National Science Review, 11, nwad285.
[8] NOAA - Global Monitoring Lab - Trends in Atmospheric Nitrous Oxide (2024). Link: https://gml.noaa.gov/ccgg/trends_n2o/
[9] U.S. EPA (2024).Understanding Global Warming Potentials. https://www.epa.gov/ghgemissions/understanding-global-warming-potentials#:~:text=Nitrous%20Oxide%20(N2O,Sinks%20uses%20a%20different%20value.)
[10]1017. Forster, P., T. Storelvmo, K. Armour, W. Collins, J.-L. Dufresne, D. Frame, D.J. Lunt, T. Mauritsen, M.D. Palmer, M. Watanabe, M. Wild, and H. Zhang, 2021: The Earth’s Energy Budget, Climate Feedbacks, and Climate Sensitivity. In Climate Change 2021: The Physical Science Basis. Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change [Masson-Delmotte, V., P. Zhai, A. Pirani, S.L. Connors, C. Péan, S. Berger, N. Caud, Y. Chen, L. Goldfarb, M.I. Gomis, M. Huang, K. Leitzell, E. Lonnoy, J.B.R. Matthews, T.K. Maycock, T. Waterfield, O. Yelekçi, R. Yu, and B. Zhou (eds.)]. Cambridge University Press, Cambridge, United Kingdom and New York, NY, USA, pp. 1017, doi:10.1017/9781009157896.009
[11] Inger, M., Moszowski, B., Ruszak, M., Rajewski, J., & Wilk, M. (2020). Two-Stage Catalytic Abatement of N2O Emission in Nitric Acid Plants. Catalysts, 10(9), 987.
[12] Minqi Liang, Zheyan Zhou, Peiyang Ren, Han Xiao, Xu-Ri, Zhongmin Hu, Shilong Piao, Hanqin Tian, Qing Tong, Feng Zhou, Jing Wei, Wenping Yuan, Four decades of full-scale nitrous oxide emission inventory in China, National Science Review, Volume 11, Issue 3, March 2024, nwad285, https://doi.org/10.1093/nsr/nwad285
Get the latest delivered to your inbox
Sign up to our newsletter for the Calyx News and Insights updates.
